Home > Products & Applications > Pressure Sensitive Labels > Vial & Syringe Labels
Labels

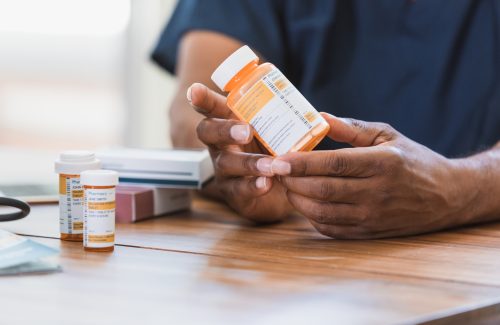
Vial & Syringe Labels — Precision, Compliance & Durability
At ADDEV Materials Healthcare, we understand that vial and syringe labels are far more than simple identifiers—they are essential to patient safety, regulatory compliance, and reliable supply chain traceability. These labels must clearly communicate critical information such as dose, concentration, lot number, and expiration date, and they must remain legible and securely adhered through the most demanding environments, including cold chain storage, sterilization processes, and chemical exposure. That’s why our pressure-sensitive label solutions are engineered for durability, clarity, and performance, integrating features like high-quality printing of barcodes and security elements such tamper-evident technologies to support both clinical and commercial pharmaceutical applications.
Here’s how ADDEV Materials Healthcare delivers superior vial and syringe labeling:
Advanced Printing Technologies
Variety of Materials & Adhesives
Precision Converting
Clean Room Manufacturing

Commercial Pharmaceutical Products
Labels for injectable drugs, biologics, and vaccines must remain readable from fill- finish through distribution and final use. They must support automated application, withstand abrasion during shipping, and maintain crisp barcodes for traceability.
We engineer pharma-grade label constructions with high-durability films, low-profile adhesives, serialization-ready print formats, and tight color control, all produced in ISO 13485:2016 environments to ensure consistency at scale
Sterile Barrier & Cleanroom Environments
Labels used inside aseptic or particulate-controlled environments must survive EtO, gamma, or steam sterilization, resist disinfectants, and stay adhered during frequent handling. They must perform without shedding particulates or compromising sterile barrier systems.
Our cleanroom converting, FDA-registered processes, and sterilization-resistant materials allow us to produce labels that maintain adhesion, legibility, and compliance in high-purity environments, including Class 7 and Class 8 cleanrooms.

ADDEV Materials Healthcare maintains a rigorous Quality Management System designed to meet the stringent expectations of the pharmaceutical industry. Our operations are certified to ISO 13485:2016 and backed by FDA registration and cGMP compliance under 21 CFR parts 210, 211, and 820, standards essential for pharmaceutical packaging, labeling, and medical components. Production takes place in ISO Class 7 and Class 8 cleanrooms, ensuring controlled environments for sensitive pharma applications. We maintain full raw material and lot traceability, conduct regular internal audits, and continuously improve our processes based on customer requirements, regulatory expectations, and quality data.
Materials Engineered for Medical Bottle & Container Labeling
Medical bottle and container labels rely on materials engineered for durability, chemical resistance, and regulatory performance. At ADDEV Materials Healthcare, we use high-performance films, specialty papers, and medical-grade adhesive systems that withstand sterilization, alcohol-based cleaners, moisture exposure, and the demanding conditions of pharmaceutical packaging. Constructions can include PET films for superior strength, polypropylene for flexibility and conformability, and adhesive technologies designed for curved surfaces and challenging plastics often used in drug delivery and diagnostic containers. To help teams specify the right material stack-up, our Material Finder allows users to explore a wide range of films, foils, papers, and adhesives tailored for medical labeling applications.
Explore it here: https://addevhealthcare.com/material-finder/
To get started, reach out to our team at 262.255.6150 or email info.amh@addevmaterials.com to discuss your vial or syringe label project.
We can support you through early design, prototyping, pilot runs, and full validation. Once your design is finalized, our team will help scale production and seamlessly integrate your labels into your supply chain.
Call us 262.255.6150 or send a message to info.amh@addevmaterials.com TODAY to connect with a specialist about your custom product.